Abstract
INTRODUCTION:Somatic mutations in the isocitrate dehydrogenase 1 (IDH1) gene occur in 6-10% of patients with acute myeloid leukemia (AML). The resulting aberrant IDH1 metabolic enzyme promotes the accumulation of the epigenetically active oncometabolite D-2-hydroxyglutarate (2-HG). Ivosidenib (AG-120) is a potent, selective, oral small molecule inhibitor of the mutant IDH1 (mIDH1) protein. Clearance of mIDH1 has previously been observed, using a next generation sequencing (NGS) technique with a lower limit of detection of 1%, in patients treated in the dose escalation phase of the single-agent phase 1 study of ivosidenib in patients with advanced mIDH1 hematologic malignancies (ClinicalTrials.gov NCT02074839; DiNardo CD et al. Blood 2016:128[22]Abs1070). For patients in the expansion phase (ivosidenib 500 mg once daily), we employed a digital PCR technique that is 50-fold to 100-fold more sensitive to assess the depth of IDH1 mutation clearance. In addition, we conducted baseline genetic profiling using NGS to determine whether a pattern of co-occurring mutations predicts response.
METHODS: The mIDH1 variant allele frequency (VAF) was assessed in bone marrow mononuclear cells (BMMCs) from patients in the expansion phase using BEAMing Digital PCR Technology (Sysmex-Inostics Inc.), which has a lower limit of detection for mIDH1 of 0.02-0.04%. Deep IDH1 mutation clearance (IDH1-MC) was defined as reduction in the mIDH1 VAF to below the limit of detection using this assay for at least 1 on-treatment time point. In addition, baseline co-occurring mutations were identified with a 95-gene NGS panel targeted to hematologic malignancies. This assay reproducibly detects single nucleotide variants and small insertions/deletions at allele frequencies of >5% (Kluk MJ et al. J Mol Diagn 2016:18;507).
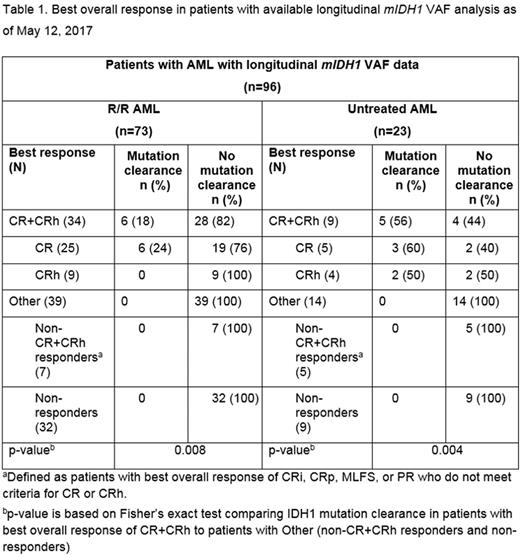
RESULTS: As of May 12, 2017, longitudinal mIDH1 VAF data from BMMCs (baseline and at least 1 on-treatment time point) were available for a total of 96 patients treated in the expansion phase of the study, including 73 of 102 patients with relapsed or refractory (R/R) AML who received their first dose of ivosidenib at least 6 months prior to the analysis cut-off date, and 23 of 25 patients with previously untreated AML (Table 1). Following treatment with ivosidenib, IDH1-MC was observed in 6 of 25 (24%) patients with R/R AML and 3 of 5 (60%) patients with untreated AML who achieved complete remission (CR; according to modified IWG criteria 2003) and for whom samples were available for analysis. IDH1-MC was also observed in 2 of 4 patients with untreated AML who achieved CR with partial hematologic recovery (CRh; defined as CR except absolute neutrophil count >0.5 × 109/L [500/µL] and platelet count >50 × 109/L [50,000/µL]). By contrast, IDH1-MC did not occur in any of the studied patients (0/39 with R/R AML and 0/14 with untreated AML) who did not achieve a best response of CR or CRh.
Co-occurring mutation results from baseline whole bone marrow samples were available for a total of 126 patients from the expansion phase, including 101 patients with R/R AML and 25 patients with untreated AML. These analyses demonstrated that a lower frequency of receptor tyrosine kinase pathway mutations and a lower co-mutational burden were observed in patients whose best response was CR or CRh compared with non-CR+CRh responders or non-responders. A relationship between baseline mIDH1 VAF by NGS and clinical response was not observed.
CONCLUSIONS: These data demonstrate that ivosidenib monotherapy results in deep IDH1 mutation clearance in a subset of patients with R/R AML and untreated AML who achieve CR or CRh. Additional analyses are required to determine the pattern of co-occurring mutations that predict response to ivosidenib and to understand the clinical impact of deep IDH1 mutation clearance.
Stone: Arog: Consultancy; Pfizer: Consultancy; Fuji Film: Consultancy; Astellas: Consultancy; Celgene: Consultancy; Abbvie: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Sumitomo: Consultancy; Amgen: Consultancy; Ono: Consultancy; Agios: Consultancy. Choe: Agios: Employment, Equity Ownership. Zhang: Agios: Employment, Equity Ownership. Wang: Agios: Employment, Equity Ownership. DiNardo: AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Stein: GSK: Other: Advisory Board, Research Funding; Novartis: Consultancy, Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Research Funding; Celgene Corporation: Consultancy, Other: Travel expenses, Research Funding; Pfizer: Consultancy, Other: Travel expenses; Seattle Genetics: Research Funding; Constellation Pharma: Research Funding. Fathi: Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kantarjian: ARIAD: Research Funding; Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding; Pfizer: Research Funding. Attar: Agios: Employment, Equity Ownership. Wu: Agios: Employment, Equity Ownership. de Botton: Servier: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Celgene Corporatation: Honoraria; Agios Pharmaceuticals, Inc.: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal